Quinta Analytica is a global provider of high quality services in the field of drug testing, clinical studies and bioanalytical services, regularly audited by the Czech State Institute for Drug Control (SÚKL); it is GMP, GLP and GCP certified and has successfully passed 10 FDA audits.
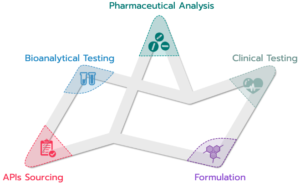
The services of Quinta Analytica include:
- Pharmaceutical Analyses: research, development and validation of methods, quality control, stability studies
- Clinical Trials: complete clinical (Phase I) study services include the actual clinical, bioanalytical, pharmacokinetic and statistical evaluation, as well as bioequivalence, pharmacokinetic and pharmacodynamic studies conducted in a fully-equipped facility with 60 hospital beds and Bioanalytical Lab.
- Formulation: Design and Optimization of formulations for dosage forms, Stability of the products, Bioavailability of the active ingredient, Chemical and physical parameters of the dosage form, Pre-formulation, publication research, Design and formulation of the dosage form (solid, semi-solid, liquid forms), Design of a suitable packaging material for the given dosage form, Accelerated stability and Natural stability studies.
- Bioanalytical Lab: Analytical service for clinical and pre-clinical studies including statistical evaluation, featuring state-of-the-art equipment, like HPLC/MS/MS devices to determine active substances and metabolites in different biological matrices and nine modern LC/MS/MS devices with triple quadrupoles, six of which with a 2-channel HPLC system. The capacity of the Bioanalytical department is up to 3750 analyses of biological samples per day.
- API Sourcing: broad portfolio of active ingredients from reliable and approved manufacturers guaranteed by Quinta Analytica
- Sourcing or synthesis of impurities, standards, metabolites.
- GMP Packaging / Labelling for stability studies and clinical testing
- Module 3 audit
- IMPD Compilation
- Monitoring of studies
- Pharmacovigilance
- Regulatory affairs
- Registrations of generics in Russia
- …And more!
Please enquire with us for more information through our contact form or send us an e-mail.